If you own or operate a construction supply outlet, you may be eligible to supply ICCONS range of construction fastening supplies at wholesale prices!
- Log In
- Sitebox
- Contact
- Find a dealer
-
Anchoring
Anchoring
- Screw Bolts
- Thru-Bolt Stud Anchors
- FM753 - Heavy Duty Through Anchors
- ATS-EVO Heavy Duty Anchors
- STA-EVO Heavy Duty Anchors
- Hangerz
- Drop-In Anchors
- Suspension and Tie Wire Anchors
- Sleeve Anchors
- Panel Brace Anchors
- Friulsider Nylon Anchors
- Metal - Light Duty
- Nylon - Light Duty
- Plasterboard and Hollow Cavity Wall Anchors
- Plastic Wall Plugs and Spaghetti
- Strike Anchors
-
Pipe Clamps
Anchoring
-
Cast-In Anchors
Anchoring
- Shims and Other
-
Hollow Section Fittings
Anchoring
-
Adhesives
Adhesives
-
Nailing
Nailing
- Rivets
-
Screws
Screws
-
Self Drillers
Screws
- Self Drilling Bugle Head
- STET Aesthetic Architectural Tamper Resistant Screw
- Self Drilling Button Head
- Self Drilling Countersunk Head
- Self Drilling Countersunk Wing
- Self Drilling Flathead
- Self Drilling Hex Head
- Self Drilling Mushroom Head
- Self Drilling Pan Head
- Self Drilling Trade Packs
- Self Drilling Trade Tubs
- Self Drilling Wafer Head
- Self Drillers
- Type 17
- Collated
-
Decking
Screws
- Needle Point
- Chipboard
- Cyclonix
-
Painted Screws
Screws
- Painted - Needle Point Hex Head
- Painted - Self Drilling Button Head
- Painted - Self Drilling Flathead
- Painted - Self Drilling Hex Head
- Painted - Self Drilling Multiseal - 14 Gauge
- Painted - Self Drilling Trade Packs
- Painted - Self Drilling Trade Tubs
- Painted - Self Drilling Wafer Head
- Painted - Type 17 Cyclonix - 14 Gauge
- Painted - Type 17 Hex Head
- Painted Screws
- Muro - Collated Screw Solutions
-
Self Drillers
-
Drilling
Drilling
- Drilling - SDS Plus
- Drilling - SDS Max
- Dustless Drilling
-
Drilling Power Tool Accessories
Drilling
- Countersinking / Pre-Drilling Accessories
- Impact Fastdrive - Bit Holders
- Impact Fastdrive - Bit Kits
- Impact Fastdrive - Insert Bits
- Impact Fastdrive - Nut Setters
- Impact Fastdrive - Power (Double Ended) Bits
- Impact Fastdrive - Power Bits
- Standard Fastdrive - Bit Holders
- Standard Fastdrive - Insert Bits
- Standard Fastdrive - Nut Setters
- Standard Fastdrive - Power Bits
- Impact Sockets
- Drill Attachments
- Tile Bits
- Ratioquick Core Bits and Accessories
- Drilling Power Tool Accessories
- Metal / Wood / Concrete Kits
-
Diamond
Diamond
-
Tools
Tools
-
Other
Other
- Support & Downloads
- Partners
- Careers
- About
Dealer Login
The Insight: Galvanic Corrosion and Fasteners
09 May 2022
Galvanic corrosion also known as bimetallic corrosion occurs when two metals of different electric potential are brought into contact in the presence of an electrolyte, the metal with the lower potential (least noble) will form the anode while the metal with the higher potential (most noble) will form the cathode. When current flows from the anode to the cathode, a chemical reaction will take place and metal forming the anode will corrode. The higher the electric potential, the stronger the current flow and corresponding rate of corrosion. Rate of corrosion will also be influenced by the conductivity of the electrolyte i.e. fresh water, salt water etc… Galvanic corrosion however does not occur if one of the conditions is not present. If two dissimilar metals are in direct contact without the presence of an electrolyte corrosion will not occur.
All metals have an electrical potential which has been measured through research and ranked into a galvanic series chart.
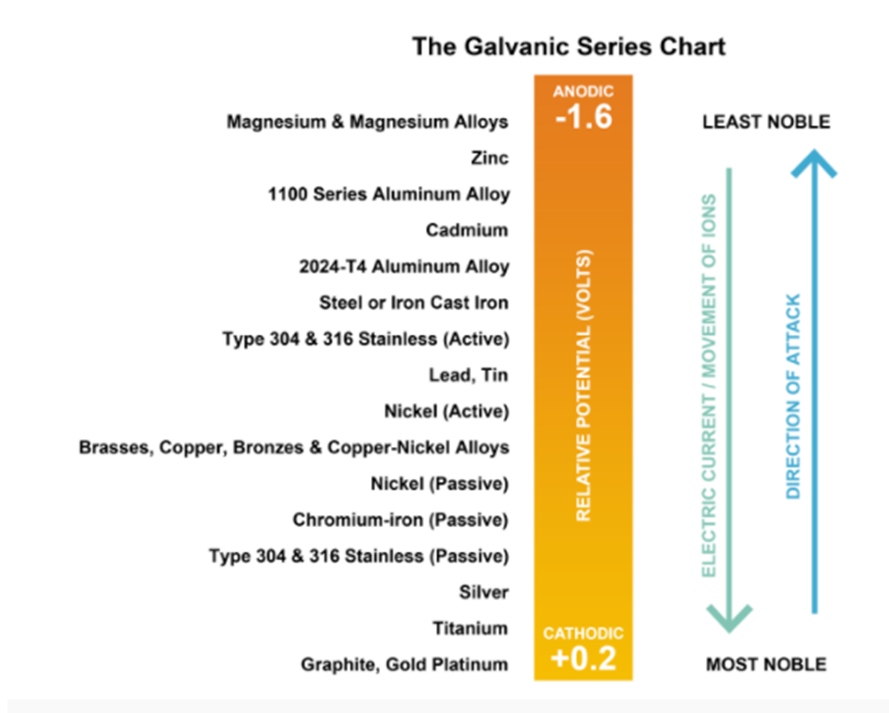
When two dissimilar metals are in contact (coupled) in the presence of a conductive solution or electrolyte (i.e. Water) electric current flows from the less noble (anodic) metal to the more noble (cathodic) metal. In any couple, the less noble metal is more active and corrodes while the more noble metal is galvanically protected. A plating metal which is less noble (lower electric potential) than the base metal it is designed to protect is usually selected. When subjected to an electrochemical reaction, the plating will corrode or sacrifice while the base metal remains protected. Once the plating has been reduced significantly, the base material will then begin to corrode. If a plating metal which is more noble is selected, the base metal would begin to corrode immediately if the plating is damaged.
For carbon steel anchors and fasteners, zinc is one of the most common plating materials used because it can be applied in a broad thickness range and because it is less noble than carbon steel. Zinc may be applied by electroplating, mechanical galvanizing, or hot dip galvanizing.
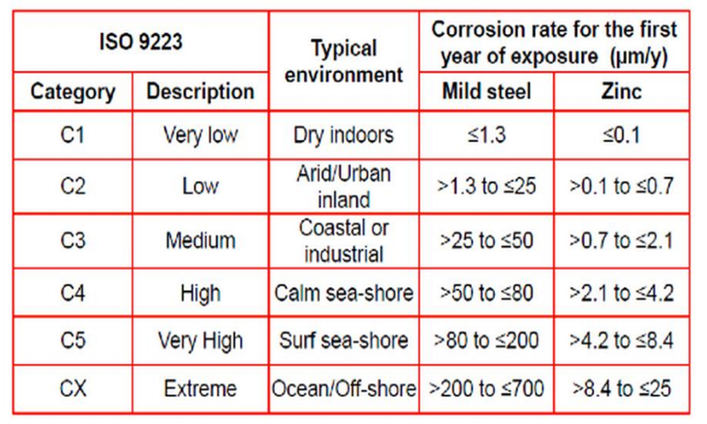
Theoretically, the life expectancy of zinc plating is based on the thickness of the plating divided by the corrosion rate. These values should only be used as a guide since actual performance will vary with local conditions, Table 1 below highlights typical corrosion rates of zinc and mild carbon steel based on corrosivity categories in accordance with ISO 9223.
As always, follow us on Instagram, Facebook and LinkedIn to stay in touch with other relevant updates!
Should you require anymore information – please don’t hesitate to get in touch with our technical experts via [email protected] or alternatively call us on 03 9706 4344.
Need further support?
Get in touch with our technical experts today
Don't forget to follow us on Facebook, Instagram and LinkedIn to stay in touch with other relevant updates!
Latest articles
-
 20 May 2025
20 May 2025ICCONS Stands Proud as a Home-Grown Brand
With many iconic Australian fixing and fastening brands now in the hands of offshore giants, ICCONS stands firm. Australian owned and operated.
Read More -
 30 April 2025
30 April 2025What's New 🔥 New Recyclable Trade Pack & Trade Tub Packaging!
ICCONS® Takes Another Step Towards Sustainability with New Recyclable Packaging!
Read More -
 5 March 2025
5 March 2025Expert Tips on Seismic Anchoring: Insights from ICCONS and KUSCH
The importance of proper selection and installation of concrete screw anchors, including ICCONS’ Thunderbolt® PRO, in seismic-rated applications.
Read More